Solutions
Innovation determines the future. QT BIO focuses on the research and development and application of isothermal nucleic acid amplification technology and has RAA technology patent authorization. Committed to creating a precise, efficient, fast, and portable nucleic acid detection platform.
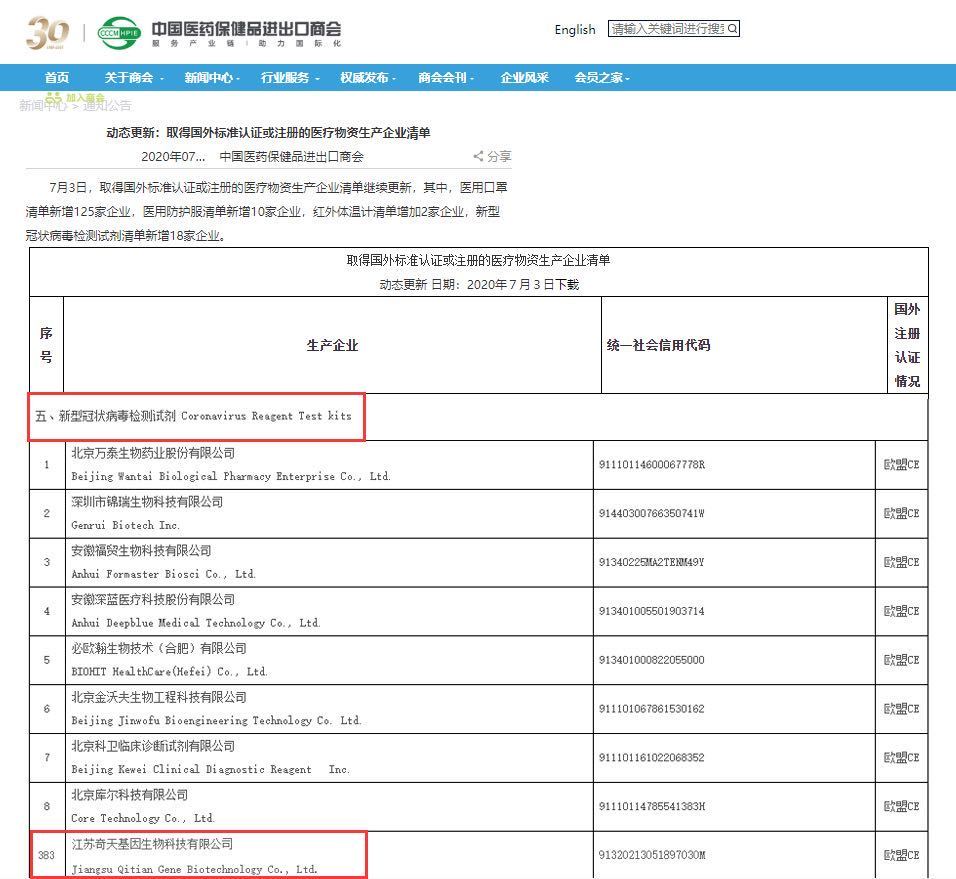
QT BIO has been approved for export of epidemic prevention materials through the Ministry of Commerce's whitelist.
Release Time:
2020-08-04
As of July 2020, a total of 394 companies have been included in the list of enterprises that produce novel coronavirus reagent test kits. QT BIO has successfully passed the Ministry of Commerce's whitelist for the export of epidemic prevention materials and is also among them. The "CE" mark is a safety certification mark, regarded as a passport for manufacturers to open and enter the European market. Passing the EU CE certification indicates that the product has met the safety requirements stipulated by the EU directive. It is a commitment from the enterprise to consumers, increasing consumer confidence in the product; it also marks QT BIO's move towards the international market and internationalization!
QT Biotech Co., Ltd.
Tel: +86-510-85385531
Mobile: +86-18921157475
Email: qt@qt-bio.com
Website: www.qt-bio.com
Address: No. 97, Xingye Building B, Linghu Avenue, Xiwu District, Wuxi City
Racing against time, guarding life safety

WeChat Account

Official Public Account

