Solutions
Innovation determines the future. QT BIO focuses on the research and development and application of isothermal nucleic acid amplification technology and has RAA technology patent authorization. Committed to creating a precise, efficient, fast, and portable nucleic acid detection platform.
Human Pathogen Detection Solution - Overall Solution for COVID-19
Release Time:
2025-05-28
Intended Use:
This kit is used for in-vitro qualitative detection of the novel coronavirus in suspected pneumonia cases, suspected clustered cases, and other individuals requiring diagnosis or differential diagnosis of novel coronavirus infection. The target is the ORFlab gene of the novel coronavirus (2019-nCoV) in throat swab and sputum samples.
Packaging Specifications:
24 tests/box, 48 tests/box, 96 tests/box
Product Components:
2019-nCoV reaction unit, Buffer IV, Magnesium acetate, 2019-nCoV positive control, negative control (G)
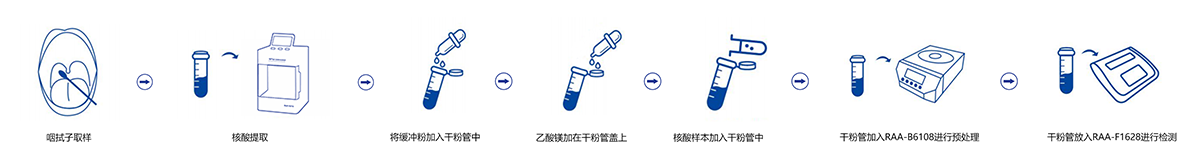
Operating Procedure
Product Features
● Detection Limit: 384 copies/ml
● High Specificity: No cross-reactivity, can detect multiple mutant strains
● Detection Time: 8-15 min
Storage Conditions and Shelf Life
The reagents should be stored at -20 ±5 "C, protected from light. The shelf life is 12 months. Unused product after opening can be stored at 2-8℃ for 3 days; after reconstitution, sample addition must be completed within 10 minutes and the test run on the machine; repeated freezing and thawing should not exceed 3 times.
Next
Next Page:
QT Biotech Co., Ltd.
Tel: +86-510-85385531
Mobile: +86-18921157475
Email: qt@qt-bio.com
Website: www.qt-bio.com
Address: No. 97, Xingye Building B, Linghu Avenue, Xiwu District, Wuxi City
Racing against time, guarding life safety

WeChat Account

Official Public Account

