News Center
News and Development
Literature Sharing: Field-Applicable Detection of Hepatitis B Virus Using Internal Controlled Duplex Recombinase-Aided Amplification Assay and Lateral Flow Dipstick Assay
Release Time:
2025-08-05
The 2025 World Hepatitis Day theme "Let’s Break It Down" emphasizes the need to simplify, scale up, and integrate hepatitis services - vaccination, safe injection practices, harm reduction and especially testing and treatment - into national health systems.
The 2025 World Hepatitis Day theme "Let’s Break It Down" emphasizes the need to simplify, scale up, and integrate hepatitis services - vaccination, safe injection practices, harm reduction and especially testing and treatment - into national health systems. HBV remains highly endemic in resource-limited regions like Southeast Asia and Africa, making simple, rapid, and portable field detection methods critical for effective control. This study presents two RAA-based field detection methods capable of completing the entire testing process within 40 minutes.
Background
HBV has a prolonged latent period in humans, often lacking early clinical symptoms, and no definitive cure currently exists. Developing efficient HBV detection methods can enhance understanding of its immunopathological mechanisms, guide clinical treatment, and improve prognosis.
Existing diagnostic methods primarily rely on serological assays, which have limited dynamic ranges and long window periods. While real-time PCR is the gold standard for HBV nucleic acid detection due to its high sensitivity and accuracy, its high cost and time-consuming nature hinder its use in resource-limited settings.
This study builds upon prior research to develop and evaluate two RAA-based field detection methods for HBV.
Technical Principle
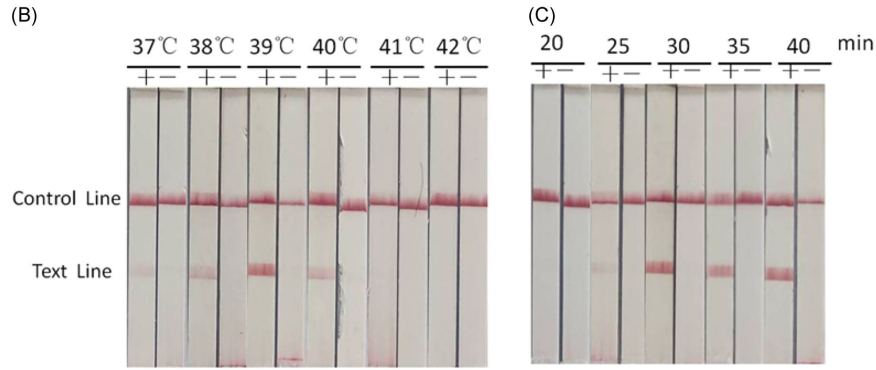
The RAA assay uses recombinase UvsX (E. coli), single-stranded DNA-binding protein (SSB), and DNA polymerase, completing the reaction at 37–42°C within 30 minutes.
Methods
Two RAA-based HBV field detection methods
1. Internally Controlled Duplex Real-Time RAA (Duplex Real-time RAA with IC)
• Dual-channel IC: Prevents false negatives (e.g., reaction inhibition). Optimized IC concentration (100 copies/μL) ensures no interference with low-concentration HBV detection.
• Rapid sample processing: Extraction-free. Serum samples are mixed with rapid extraction buffer, heated at 80°C for 3 minutes, then cooled, and directly used for testing.
• Portable device: RAA-F1620 fluorescence detector.
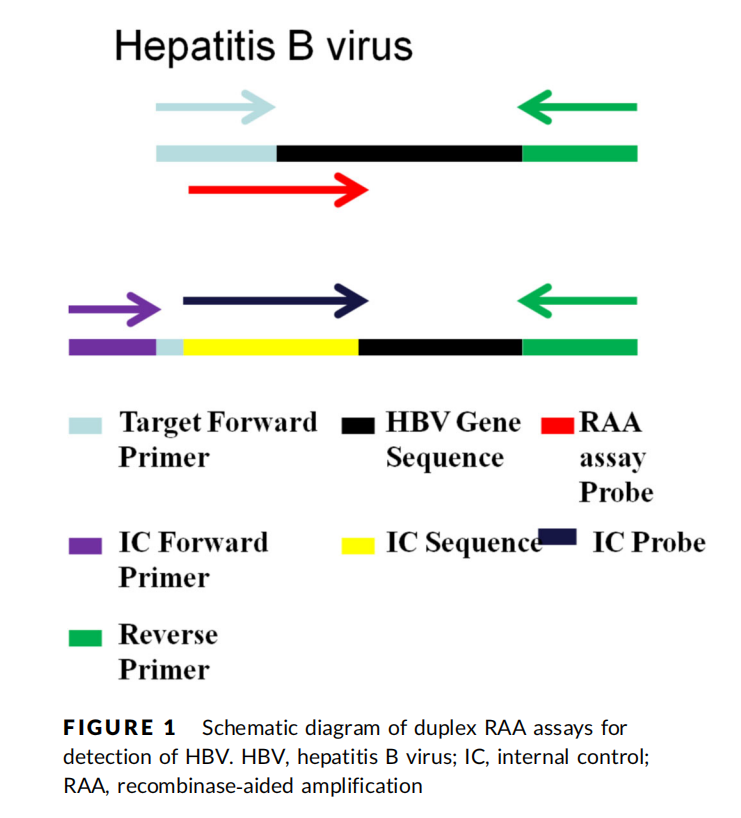
2. Lateral Flow Dipstick Assay (RAA-LFD)
• Visual interpretation: Amplified products are applied to test strips, with results read via lateral flow chromatography.
• Instrument-free: Results are visible to the naked eye.
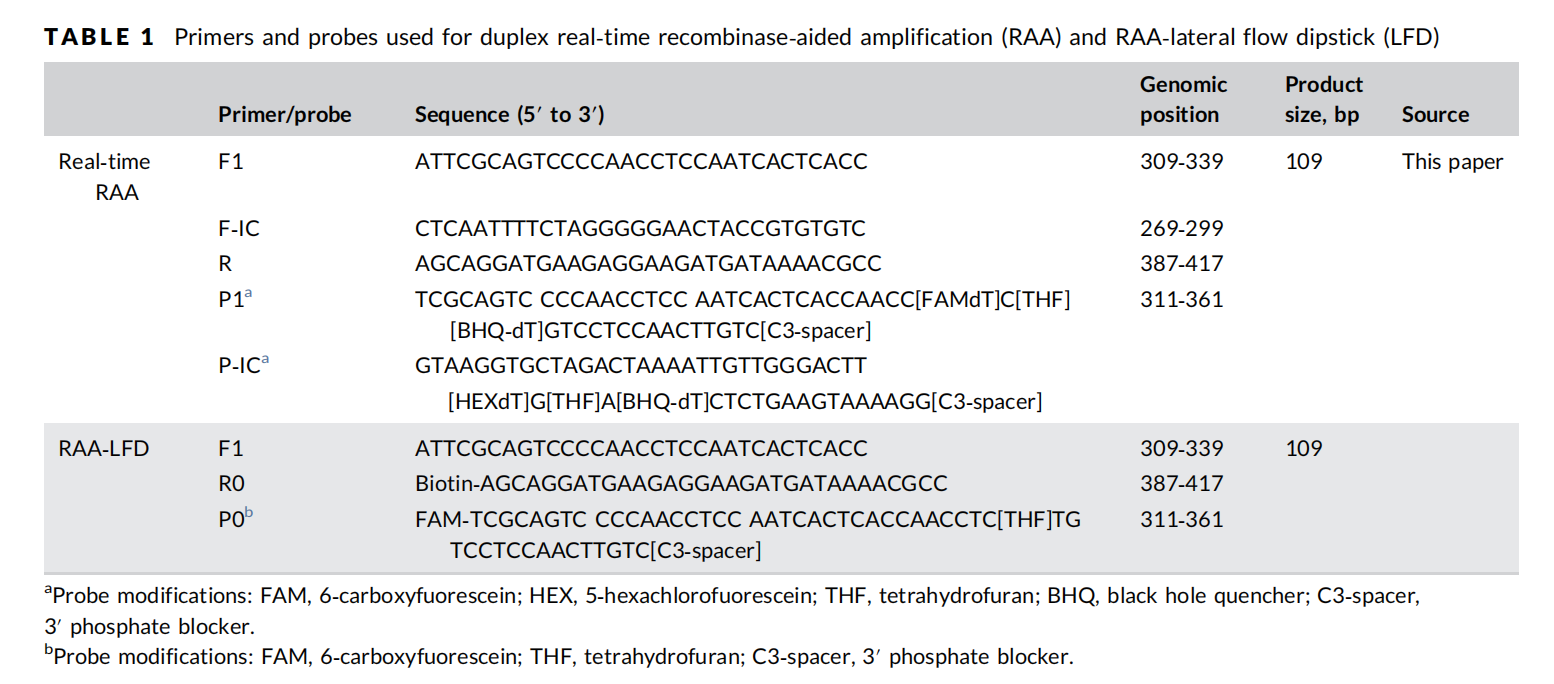
Primer & Probe Design
Performance Evaluation
Duplex real‐time RAA assay
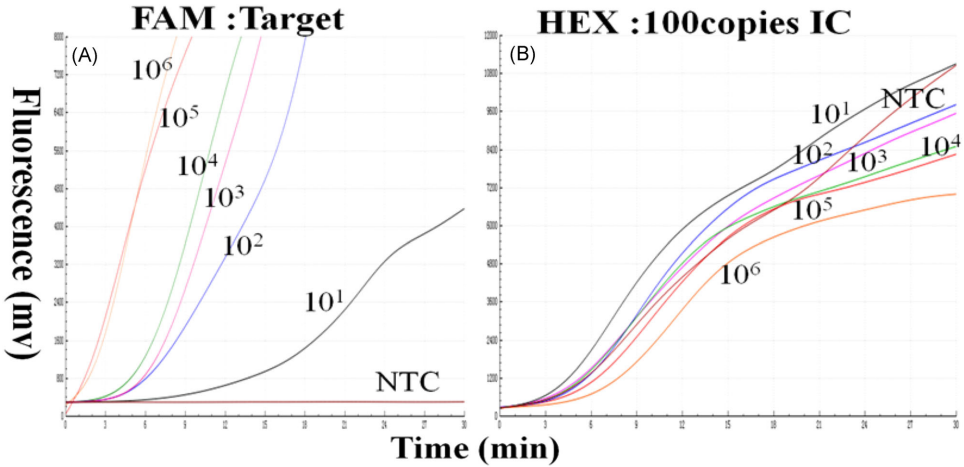
• Tested using qPCR standards (106 to 10 IU/mL). At optimal IC concentration (100 copies):
• 106–102 IU/mL: 8/8 replicates positive.
• 10 IU/mL: 5/8 replicates positive.
• Limit of detection (LOD): 10–100 IU/mL.
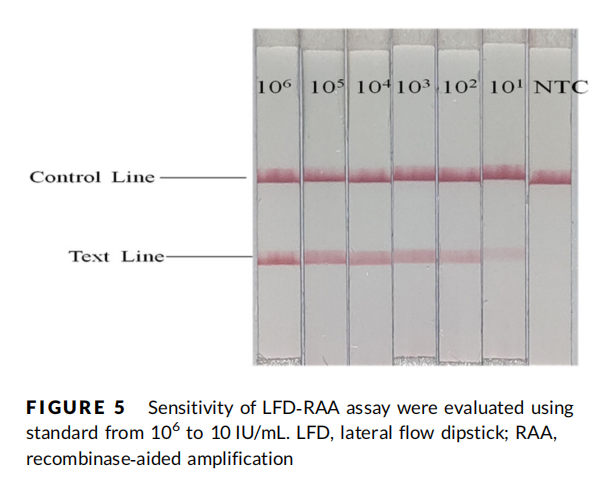
RAA-LFD
• 106–102 IU/mL: 3/3 replicates positive.
• 10 IU/mL: 1/3 replicates positive.
• Limit of detection (LOD): 10–100 IU/mL.
Specificity
Both methods showed 100% specificity, with no cross-reactivity in 15 serum samples of healthy donors, , five EBV, and five CMV‐positive serum samples.
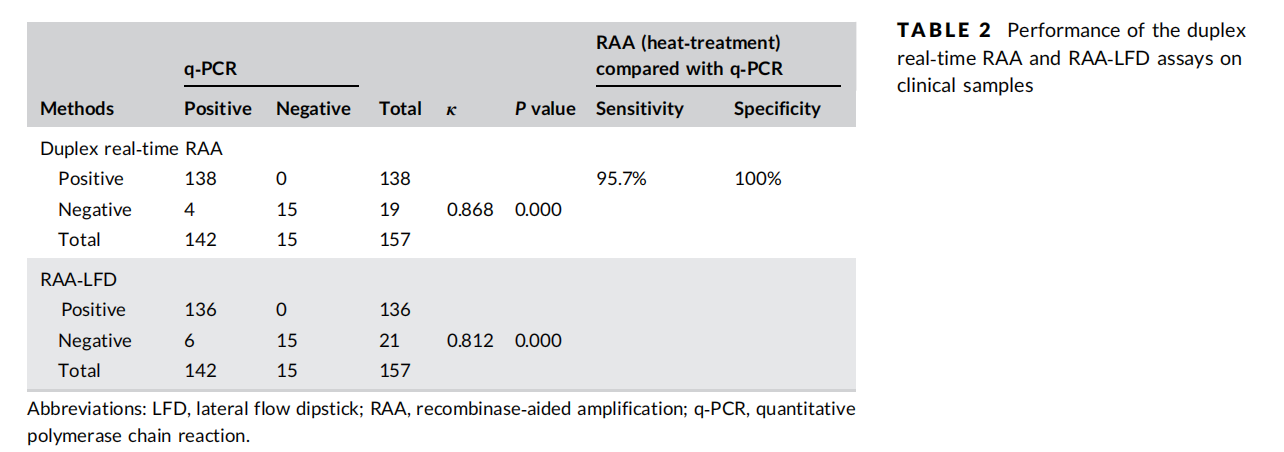
Clinical Validation (157 HBsAg-positive patient sera)
Reference method: qPCR (viral load <10 IU/mL = negative).
qPCR detected 142 positives (range: 1.80×10–5.94×108 IU/mL).
- Duplex Real-time RAA with IC
• Sensitivity: 97.18% (138/142); Specificity: 100%.
- RAA-LFD
• Sensitivity: 95.77% (136/142); Specificity: 100%.
Note: When using traditional DNA extraction, Duplex Real-time RAA detected all positives.
Conclusion
Both RAA-based methods enable accurate, rapid, and portable HBV detection without traditional DNA extraction (requiring only 3-minute heating). Their simplicity, visual readout, and portability make them ideal for point-of-care testing (POCT) in resource-limited settings (e.g., primary care clinics, remote areas, and field epidemiology). These tools facilitate rapid screening, diagnosis, and outbreak surveillance, supporting timely intervention, transmission control, and improved patient management.
QT Biotech Co., Ltd.
Tel: +86-510-85385531
Mobile: +86-18921157475
Email: qt@qt-bio.com
Website: www.qt-bio.com
Address: No. 97, Xingye Building B, Linghu Avenue, Xiwu District, Wuxi City
Racing against time, guarding life safety

WeChat Account

Official Public Account

