News Center
News and Development
Literature Sharing: Clinical Validation of Two Recombinase-Based Isothermal Amplification Assays (RPA/RAA) for the Rapid Detection of African Swine Fever Virus
Release Time:
2025-08-28
African Swine Fever (ASF), caused by the African Swine Fever Virus (ASFV), is a devastating infectious disease affecting domestic and wild pigs.

African Swine Fever (ASF), caused by the African Swine Fever Virus (ASFV), is a devastating infectious disease affecting domestic and wild pigs. It has significant negative socioeconomic impacts on the global pig industry and food security. The World Organisation for Animal Health (OIE) classifies it as a notifiable disease. To date, there is no effective vaccine or treatment for ASF. Early detection and rapid diagnosis are of critical importance for controlling the spread of ASF.
Methods
Recombinase-based isothermal amplification assays, such as Recombinase Polymerase Amplification (RPA) developed by TwistDx (Cambridge, UK) and Recombinase-Aided Amplification (RAA) developed by Qitian Gene (Wuxi, China), are emerging as molecular tools for the rapid, specific, and highly sensitive identification of various pathogens. In this study, we aimed to investigate whether RPA and RAA can serve as tools for on-site rapid detection and low-concentration sample testing of ASFV. A total of 152 clinical samples, previously well-characterized by OIE-recommended qPCR, were selected for this study, including 20 weak positive samples (Ct value ≥ 30).
Experimental Design
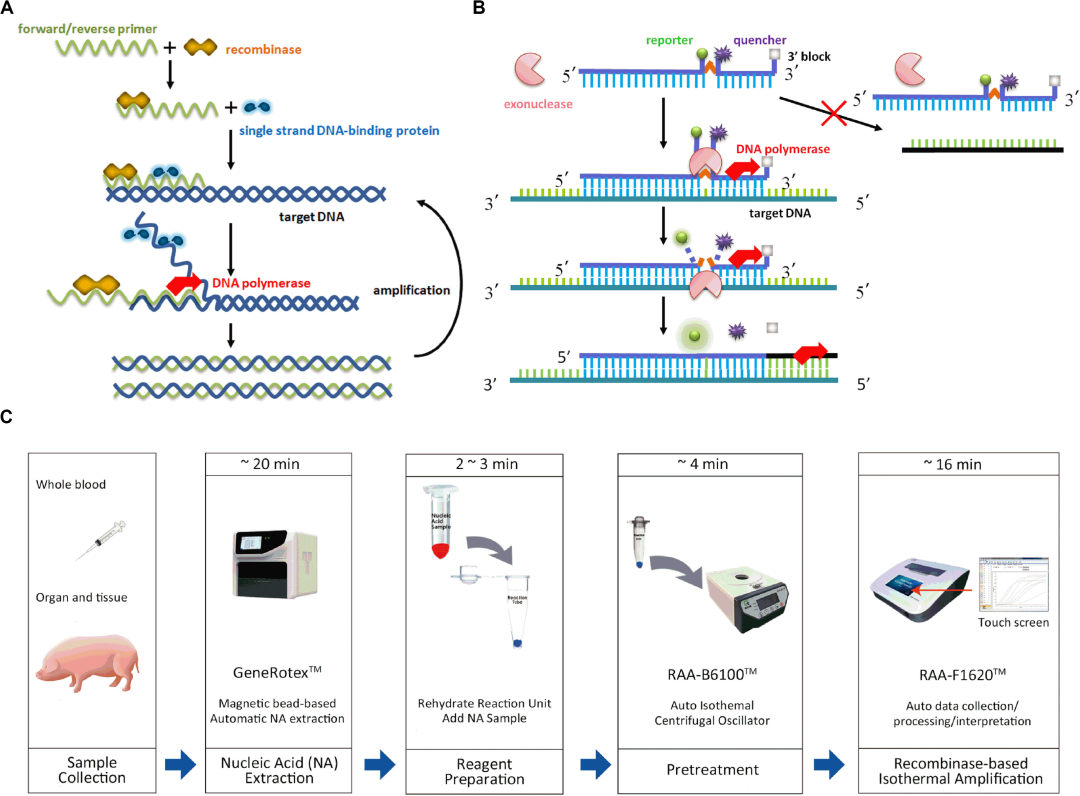
Schematic of recombinased-based assay and process of sampling, nucleic acid extraction and amplification.
(A) Recombinased-based reaction mechanism. (B) Probe principle. (C) Whole process of recombinased-based (RPA/RAA) detection.
Experimental Data
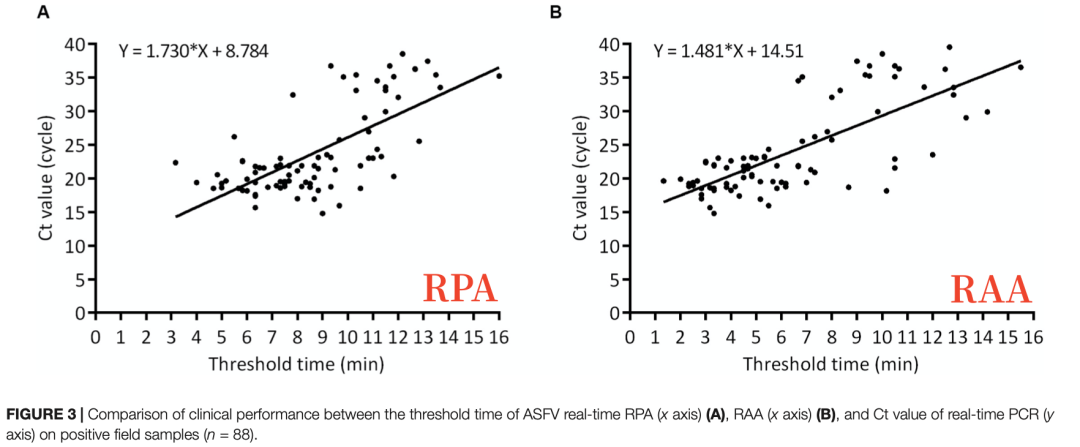
Results
Testing results on various clinical samples showed that the diagnostic consistency between RPA, RAA, and the OIE-recommended real-time quantitative PCR method was high, with kappa values of 0.960 and 0.973, respectively. Compared to real-time PCR, both RPA and RAA demonstrated 100% specificity (94.40%∼100%, 95% CI), while their sensitivities were 96.59% (90.36%∼99.29%, 95% CI) and 97.73% (92.03%∼99.72%, 95% CI), respectively. Comparison of Experimental Data: RAA demonstrated both higher sensitivity and specificity compared to RPA.
The data indicate that the developed recombinase-based amplification assays (RPA/RAA) have the potential to be equipped with field-deployable instruments, providing a highly sensitive and specific platform for rapid and reliable detection of ASFV. This is particularly valuable for ASF screening and monitoring in resource-limited settings.
QT Biotech Co., Ltd.
Tel: +86-510-85385531
Mobile: +86-18921157475
Email: qt@qt-bio.com
Website: www.qt-bio.com
Address: No. 97, Xingye Building B, Linghu Avenue, Xiwu District, Wuxi City
Racing against time, guarding life safety

WeChat Account

Official Public Account

